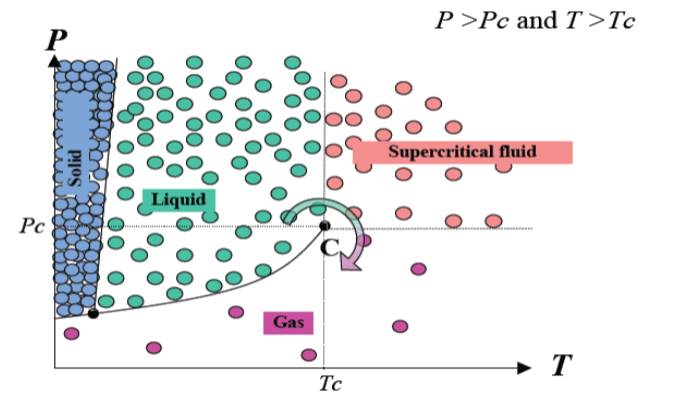
A fluid is called “supercritical” when both its pressure and temperature are over its critical pressure and temperature. It is monophasic and exhibits specific properties, different from those of liquids and gases.
Supercritical fluids have a high solvent power vis-à-vis many compounds, at the difference with compressed gases. This solvent power can be easily modified by changing the pressure, what permits to design very selective processes leading to high-quality products.
Often used as supercritical fluid, Carbon Dioxide presents numerous advantages :
- Non toxic
- Non-flammable
- Inexpensive
- Low chemical reactivity
- Critical conditions easy to reach
- High diffusivity.
Supercritical fluids have numerous applications from food industries to pharmaceuticals, cosmetic use …



